
A One-Stop Solution for High-Speed Production with Full FDA Compliance: A Packaging Manufacturer Case
Client Background
A medical packaging solution provider for export
Challenge
1. The original Japanese PLC was a closed loop control system, which failed to meet the standard for production capacity in motion control. Alternative German solutions could meet demands in production capacity but the costs of motion control systems exceeded the project budget.
2.The client’s products for export fell in the category where the FDA (Food and Drug Administration) 21 CFR Part 11 regulations applied to. As specified, all requirements are to be adhered to for open and closed electronic record systems, and those when building connections between electronic signatures and records. There were few solutions on the market at that moment that met all the requirements.
Solution
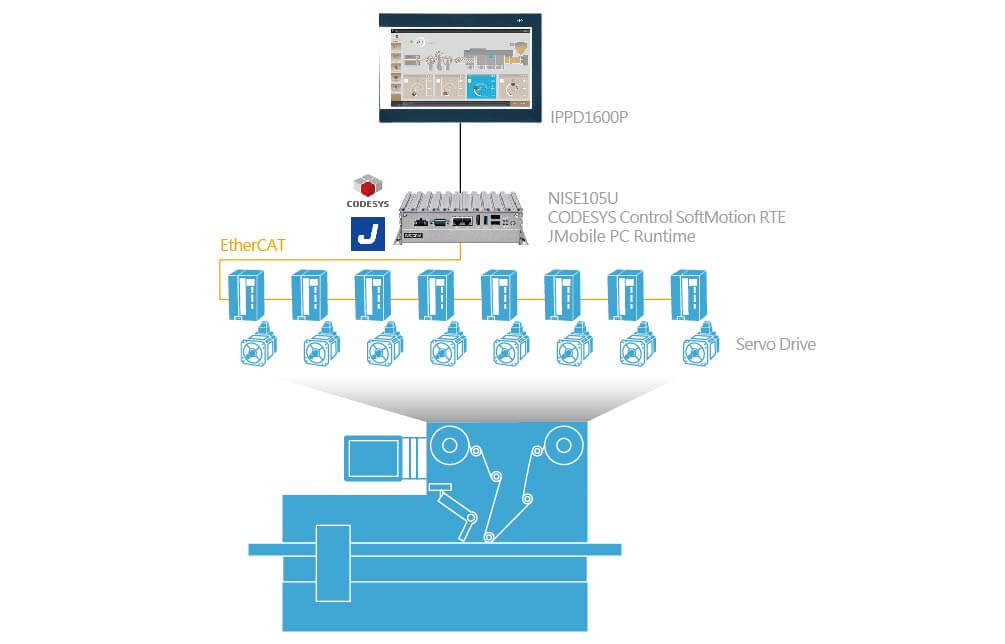
- IIoT gateway(IIoT Gateway):NISE105U
- Motion Control Software:CODESYS Control SoftMotion RTE
- Visualization Software : JMobile PC Runtime
-Display: IPPD1600P
1.A pair of servo and motor by Shilin Electronics was adopted, with the orchestration of controller NISE105U and software CODESYS Control SoftMotion RTE, to enable 8-axis motion control. In packaging operation, it could adjust the tension of packaging material rolls and fit the length of enveloping material to the size of objects for optimal results. The NexAIoT solution stroke a great balance between high performance, stable operation and system reliability.
2. JMobile PC Runtime, a software coming with NISE105U, supports data output to the 15.6” panel PC IPPD1600P, convenient for visualization. JMobile PC Runtime is fully FDA 21 CFR Part 11 compliant, meeting all requirements to open and closed electronic record systems, and those to building connections between electronic signatures and records.
Results & Benefits
1. NISE105U, an EtherCAT multi-axis controller by NexAIoT, is not just high-performance yet reliable to deliver real-time computing, but also compatible with major industrial Ethernet protocols. It saves facility investment as well as extra efforts on complex cabling in installation, helping the client improve productivity by half, from 300 pcs to 450 pcs per minute.
2. Among the few solutions on the market that are FDA 21 CFR Part 11 compliant, NexAIoT’s solution NISE105U has more to offer. The accompanied software JMobile PC Runtime is easily accessible in any browser, complete with HTML 5, JavaScript, and MQTT support. On top of the benefits on decision making and remote management, the NexAIoT solution is 100% FDA approved to open up unlimited market prospects in America and Europe for solution providers.

[Case Study] Packaging Manufacturer Case

